In the wake of their oversubscribed capital raising effort, clinical stage pharmaceutical company Pharmaxis (ASX: PXS) is forging ahead with research into their anti-fibrotic drug pipeline.
For those unfamiliar with their work, Pharmaxis is developing drugs based on their proprietary amine-oxidase technology. These drugs work by inhibiting an enzyme pathway that is key in the process of fibrosis and scar formation. The Company has two lead assets currently in clinical trials- PXS-5505 and PXS-6302.
PXS-5505 is currently in Phase 2 clinical trials for the treatment for rare blood cancer myelofibrosis as well as liver cancer. There is also preclinical work ongoing looking into the use of PXS-5505 in other indiciations like brain cancer and pancreatic cancer.
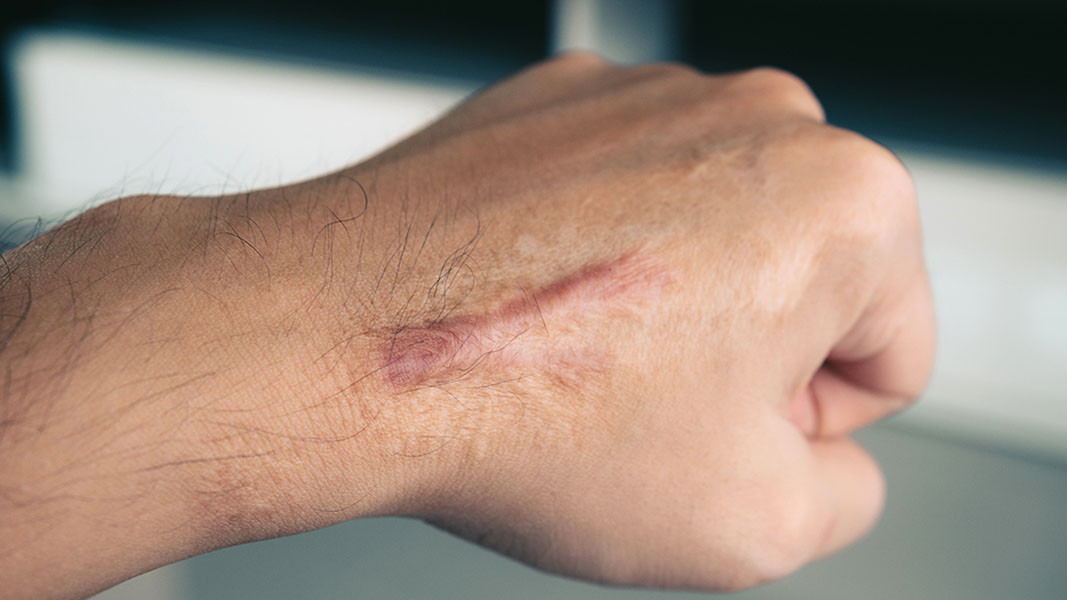
PXS-6302 is based on the same technology, but for a vastly different purpose. The topical cream is the first of its kind anti-skin scarring solution.
With reach far beyond their Sydney offices, the Company has multiple engagements with world renowned researchers and opinion leaders who collaborate on research and development of the suite of Pharmaxis drugs.
The Company’s relationship with Professor Fiona Wood AM in particular has been exceptionally beneficial with Professor Wood seeing anti-skin scarring drug PXS-6302 through to clear Phase 1a trials back in August 2021.
With pharmacological approaches to skin scarring currently quite limited, the drug could carve itself a space in the dermatology market as a revolutionary new approach to the treatment and prevention of scars. Developed on Pharmaxis’ proprietary amine oxidase platform, the topically applied drug is designed to eliminate skin scarring whilst reducing the incidence of comorbidities that arise with scarring.
Since passing the safety trial with flying colours PXS-6302 will now be trialled in 50 patients with established scarring. The Phase 1c study will run for 3 months and will report on safety, tolerability and effects of scar structure and appearance. Results are expected in 2H 2022.
Scars can be debilitating both in mentally and physically. Although the scar treatment market is fragmented, blurred between dermatology and cosmetic industries, there is ample affordability. The global scar treatment market is valued at a huge USD $20.6 billion and is expected to grow at a compound annual rate of 10.7% to hit USD $46.3 billion by 2028. Pharmaxis however, include a very conservative USD $3.5 billion per year for acute scars in their announcement.
CEO of Pharmaxis Gary Phillips said: “We place enormous value on our collaboration with Fiona Wood and the team at UWA. They identified the opportunity of utilising our expertise in drug development and extensive knowledge of lysl oxidase enzymes to help patients with scars and have provided the clinical leadership to get this study underway. I look forward to reporting on the results later this year for a project that has significant potential both clinically and commercially.”
Professor Wood is also collaborating with Pharmaxis to conduct preclinical work on another drug from their preclinical pipeline with potential to be a treatment for tissue repair and inflammatory skin disease. Professor Wood and colleagues from the University of Western Australia and the University of Technology Sydney received a grant from the government of $590,200 to support this work.
- Parkinson’s UK backs Pharmaxis with $5 million to slow the onset of incurable disease with ‘ground breaking’ trial - September 1, 2022
- How this company is developing medtech to support Indigenous community health - August 22, 2022
- A round of ap-paws for PharmAust, changing the ruff prognosis for dogs with lymphoma - August 17, 2022












Leave a Comment
You must be logged in to post a comment.