Digital microscopic imaging company Optiscan (ASX: OIL) has gotten feedback from the US Food and Drug Administration (FDA) in relation to its Pre-Market Notification (510(k)) application for the InVivage product intended for oral tissue imaging.
FDA’s regulatory clearance for the product requires that both the fluorescent contrast agent drug and the device used to visualise microscopic structures are cleared for use in the intended clinical setting. As for being included in the De Novo submission, the Company must prove the novelty of the contrast agent and its intended application to oral tissues.
Optiscan’s CEO and Managing Director, Dr Camile Farah said, “The FDA have provided feedback to the Company that due to the first-in-class nature of the InVivage® and the novelty of its intended use, it could not evaluate substantial equivalence of our device/drug combination product due to the absence of a predicate, and as such they have deemed that the De Novo Classification Request pathway is more suitable for our purposes.”
A De Novo Classification request is a pathway to obtain FDA clearance for novel medical devices that do not have an existing device on the market that has already been cleared or approved by the FDA for similar use. When a medical device manufacturer cannot find a predicate device to compare their new device to, they can submit a De Novo request to the FDA.
Optiscan’s Chairman, Mr Robert Cooke said, “The Company’s intended De Novo submission will be directly overseen by our CEO. We are fortunate to have Dr Farah lead a future submission from its inception. His training as both a physician and pathologist, coupled with his expertise as a medical researcher will undoubtedly strengthen the Company’s submission and ensure its success. The positive working relationship established between Dr Farah and the FDA review panel will facilitate progress of the InVivage submission.”
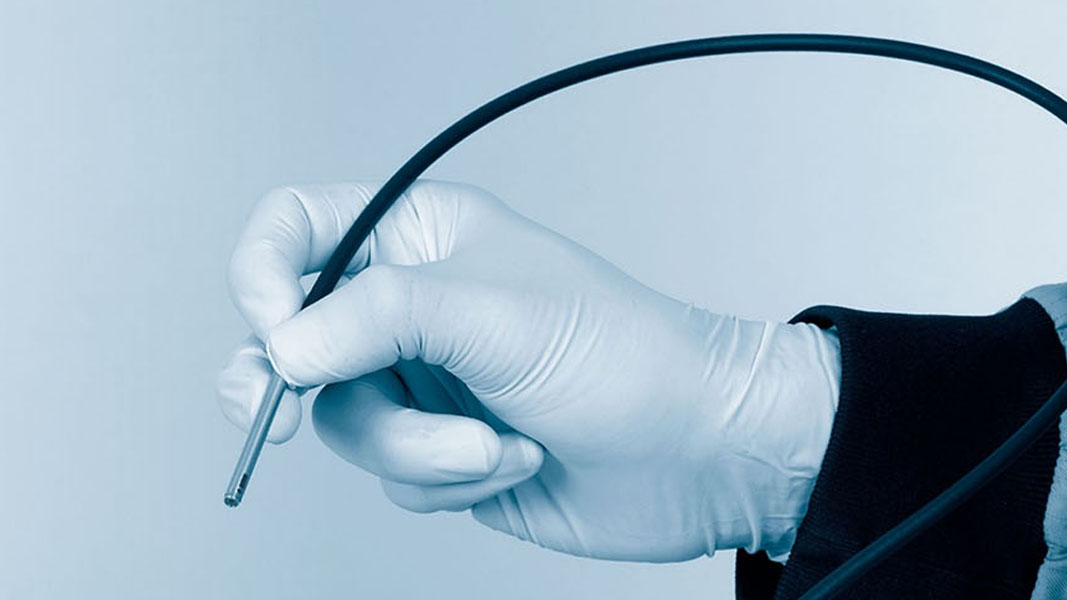
InVivage is a medical device that is used for microscopic imaging of tissues inside the body during medical procedures. It uses a specialised imaging technique called “confocal laser endomicroscopy” to produce high-resolution images of tissues in real-time. The InVivage product is intended to help doctors and surgeons visualize and diagnose a variety of medical conditions, including cancer and inflammatory bowel disease.
Even as the Company speedily moves towards a new FDA direction, Farah asserts that this was not how it was supposed to go. She added, “While this advice differs from that previously provided to the Company by the FDA in QSubmission, we are encouraged by the review process and feedback offered in our recent meetings with the FDA which have been extremely positive and productive. The review period has been time well spent as it strengthens our next submission. In addition to discussions around novelty, first-in-class, and available pathways for clearance, the FDA recommended additional studies to expedite a future De Novo submission.”
Optiscan will undertake more studies to meet FDA recommendations as quickly as possible, and they will work closely with the review team to make things go faster. They’re not just working on getting the InVivage product approved, but they’re also planning to make new tools and use them in new ways that will benefit from the FDA feedback.
- Ovanti’s iSentric signs contracts worth $14.4m with Malaysian commercial bank - June 27, 2024
- Baby Bunting fights back from retail downturn with 5-year strategy, includes Gen-Z focus and self-funded growth - June 27, 2024
- CLEO meets with US FDA to develop strategy for ovarian cancer test launch - June 26, 2024











Leave a Comment
You must be logged in to post a comment.