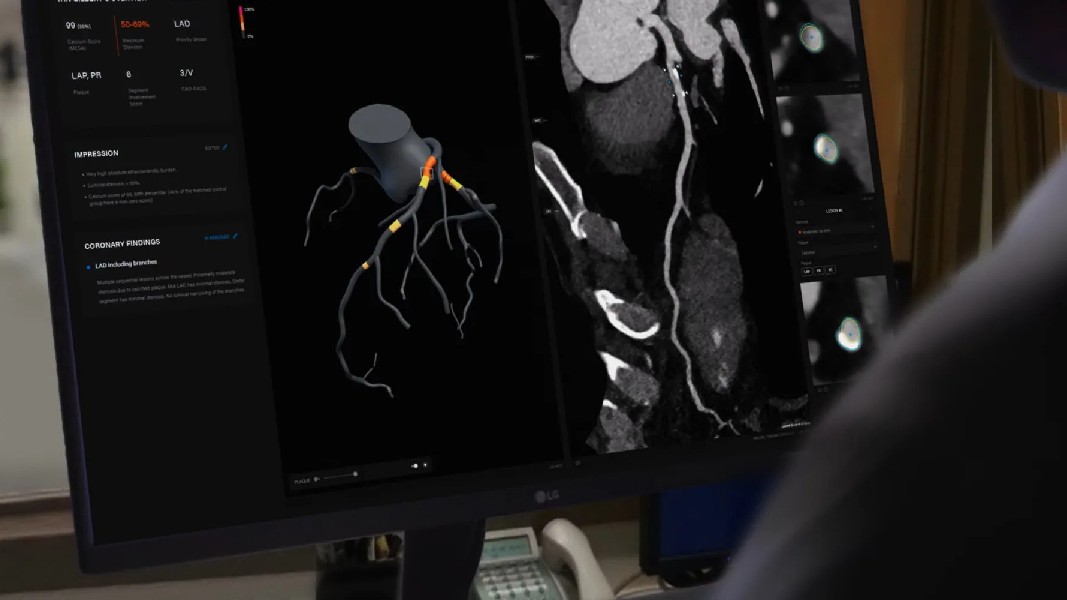
“Coronary Artery Disease. We see you.” Quite literally, healthcare company Artrya (ASX: AYA) sees your heart disease through its artificial intelligence solution Salix, which uses AI imaging to detect the intensity of your heart disease and aims to leverage its tech to access a global market alongside another jab at its FDA approval.
In June 2022, the Company revealed that it didn’t get FDA approval to sell Salix in the US markets. So, this year, Artrya will cut costs to refocus on areas of greatest immediate returns and preserve capital so that it can improve its product to sell in the US market. The Company realises that the Salix solution has immense potential in the US, given the country’s growing ageing population, Covid-driven health issues and the increased focus on health and wellness. There is more awareness of heart health, and AI has an integral role to play in that regard.
Artrya spent a significant amount on marketing the solution so that it could swiftly move into the US once the FDA approval came through. However, since that didn’t happen, it is going to redirect its efforts for the time being into three strategic areas of product refinement, regulatory preparation and revenue generation.
In Australia and New Zealand, Artrya focused on refining its SCA product after gaining feedback from its pilot site testing programme. Over 400 scans per month are being processed through the SCA product allowing for continued refinement and calibration. The feedback has been focused on data ingestion, accuracy improvement and performance improvement.
The Company also made significant progress in the United Kingdom. In October 2022, Artrya signed a contract with a commercial imaging practice ahead of regulatory approval. Plus, it signed a research contract with a National Health Service Trust Hospital to understand how efficient the Salix Coronary Anatomy product is and how it is progressing. This study is independent of regulatory approval processes and its results will set the foundation for UK sales and marketing activities.
Financially, the Company remains in a strong position with $30.5 million of cash as of 30 September 2022 and a net monthly cash burn of $1.7 million. Though the cushy cash position gives Artrya enough to commercialise well, the significant cash burn could be a cause for concern. In keeping with that, it has made fiscally responsible changes across global operations so that it could invest in product development, revenue generation, and most importantly, getting regulatory approval.
Plus, it expects the cost cutting measures to have a positive impact on its cash burn, reducing it in the short to mid term. Artrya spent over $5 million in the quarter for the development, clinical trial, regulatory expenses, commercialisation and administration of Salix. Though it is supported by federal grants and other funding options, the Company does plan on reimagining its financial priorities to set itself up for success.
- Ovanti’s iSentric signs contracts worth $14.4m with Malaysian commercial bank - June 27, 2024
- Baby Bunting fights back from retail downturn with 5-year strategy, includes Gen-Z focus and self-funded growth - June 27, 2024
- CLEO meets with US FDA to develop strategy for ovarian cancer test launch - June 26, 2024












Leave a Comment
You must be logged in to post a comment.