As the global rates of heart disease increase, countries are adopting tech solutions for early detection and prevention. That’s where Australian medical technology company Artrya (ASX: AYA) comes in. The Company reported that it had received regulatory approval for its Salix Coronary Anatomy (SCA) product in the United Kingdom.
The European Notified Body (BSI) has notified Artrya that the UKCA Class 2 Certification assessment has been completed. They will recommend certification of the Salix V2.0 Software for marketing in the United Kingdom. The BSI assessment scope includes UKCA certification per the UK Medical Devices Regulations 2002. Safe to say, Artrya’s Salix AI Coronary Software met all regulatory requirements.
Once the UKCA certificate has been issued by BSI, Artrya will get started with marketing the Salix software in the UK.
According to Artrya Managing Director and CEO John Barrington AM, the UK regulatory approval is a landmark moment for the company. He said, “Our business activities in the UK are advanced, and Artrya is well positioned to take advantage of the regulatory approval in this significant market.’’
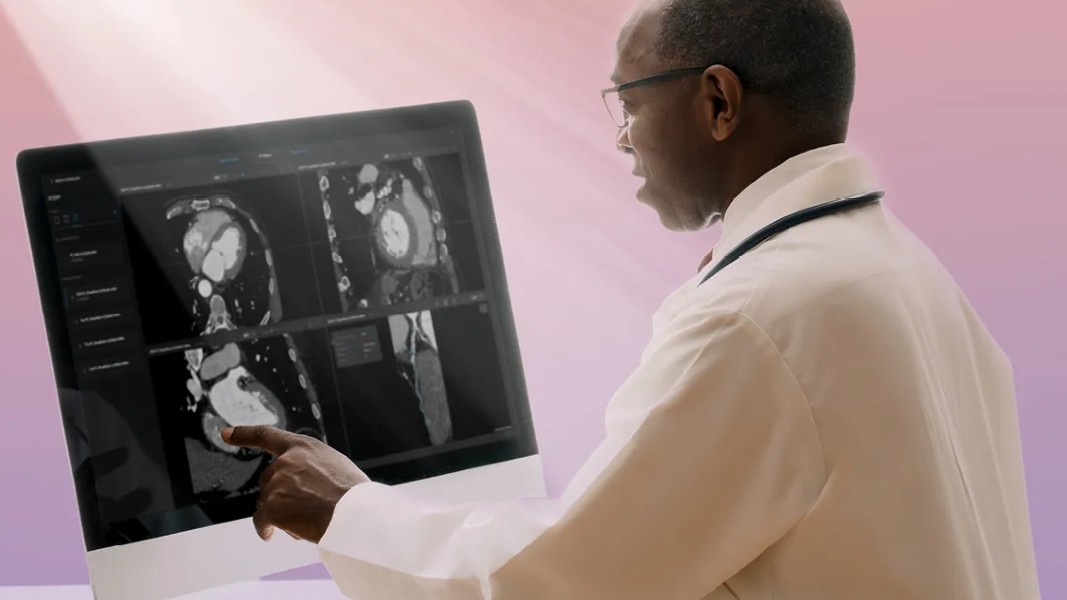
The Salix device works using artificial intelligence. It scans the body and develops a 3D heart model and coronary images. Then, it creates an AI-driven insights report in 15 minutes and locates the vulnerable plaque causing heart disease on the 3D model. The device requires minimum human intervention and speeds up the process of finding the root cause of the problem, essentially saving healthcare companies time and money.
Artrya already has a four-year contract in place to supply 1,250 National Health Service Trust Hospitals throughout the UK with the SCA product. The company can now approach these hospitals to finalise arrangements for the product rollout.
Barrington added, “This is Artrya’s biggest market opportunity to date, and we aim to take full advantage of it.” The Company also has big plans for Salix outside the National Health Service network. Other business development activities in the UK might also benefit from the SCA product within clinical practices.
The Company has been making considerable strides in Europe, in late October this year, Artrya received regulatory approval for its Salix Coronary Anatomy (SCA) product in the continent. Once it gets the EC certificate from BSI, Artrya will start marketing the Salix software in 28 European Economic Area member countries.
Besides the European region, Artrya has been pushing forth its global expansion strategy. In July, it gained the approval to sell its product in New Zealand. Currently, it is awaiting US FDA approval as the Company cuts costs and focuses on entering this market. Artrya may have increased its odds of getting US approval with a new US-based director on board. For now, however, its goal is to create a marketing strategy for Salix in the UK to set itself up for success.
- Ovanti’s iSentric signs contracts worth $14.4m with Malaysian commercial bank - June 27, 2024
- Baby Bunting fights back from retail downturn with 5-year strategy, includes Gen-Z focus and self-funded growth - June 27, 2024
- CLEO meets with US FDA to develop strategy for ovarian cancer test launch - June 26, 2024












Leave a Comment
You must be logged in to post a comment.