Med-tech developer ResApp Health (ASX: RAP) has received certification from European regulators for the world’s first smartphone-based respiratory diagnostics test.
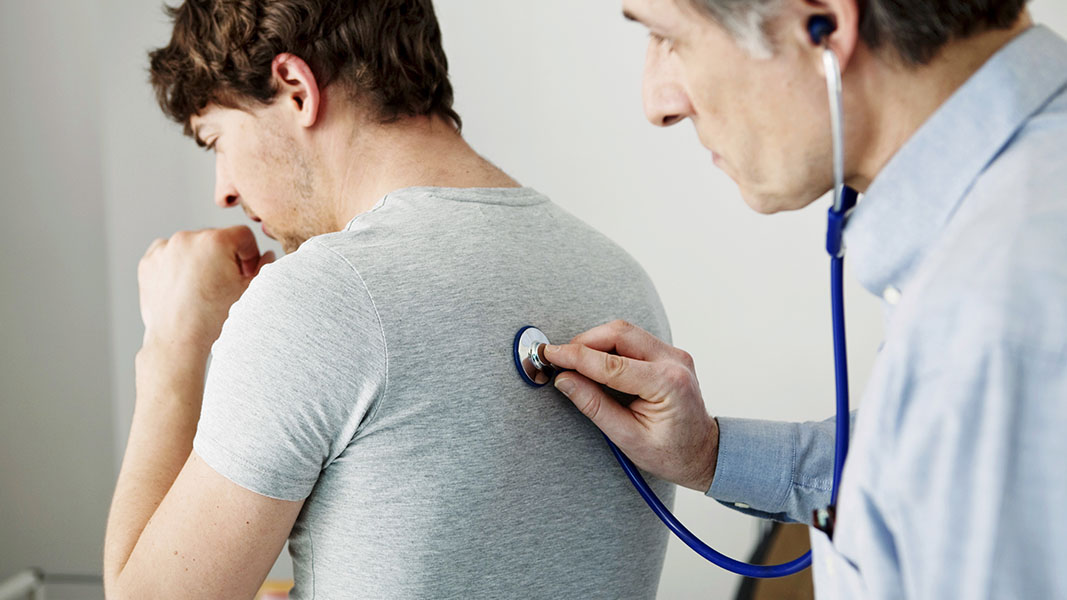
ResAppDx-EU is a mobile app which can be used by medical clinicians to diagnose respiratory conditions including tract disease, croup, pneumonia, asthma and bronchiolitis.
“We are very excited about receiving our first regulatory approval for ResAppDx-EU, our flagship acute diagnostic product,” said ResApp CEO and Managing Director Tony Keating.
“For the first time clinicians in Europe will have access to a rapid and accurate diagnostic test for the most commonly seen acute respiratory conditions in children.
“ResAppDx-EU has the potential to have far-reaching benefits in the healthcare system and we will now rapidly move ahead with our European commercialisation strategy.”
The regulatory approval was supported by a clinical trial with 585 patients which evaluated the app’s machine learning algorithms to analyse a user’s cough sounds to diagnose disease. The corresponding results were conclusive and published in peer-reviewed journal Respiratory Research.
The market for respiratory diseases is compelling, with more than 700 million doctor visits annually for respiratory complaints and ResAppDx is the only smartphone-based product in the market which does not require an external hardware component.
ResApp currently has a classification request pending in the United States with the Food and Drug Administration.
Since resumption of trade following the release of today’s announcement, shares in ResApp rose as much as 50% to an intra-day high of 22.5 cents per share.
- Harris Technology to expand refurbished tech division amid rising demand from cost-conscious Australians - April 30, 2025
- Harris Technology secures major investment from Taiwan’s FSP Technology at 100% premium - March 10, 2025
- ARC Funds acquires 30% of auzbiz Capital as latest direct-to-investor marketing venture - October 8, 2024












Leave a Comment
You must be logged in to post a comment.