It may soon be easier for prostate cancer to be diagnosed at an early stage with Telix Pharmaceuticals (ASX: TLX) having submitted their radiopharmaceutical imaging technology to the Food and Drug Administration for approval.
The New Drug Application (NDA) comes after completing clinical trials across more than 600 patients which builds on peer-reviewed clinical research conducted at top research facilities including the University of California, Los Angeles (USA), the Peter MacCallum Cancer Centre (Australia) and Heidelberg University Hospital (Germany).
“Submitting an NDA to the US Food and Drug Administration for our first product is a major commercial inflection point for the Company and follows our European submission earlier this year,” said Telix CEO, Dr Christian Behrenbruch.
“The Telix team and our advisors have done an outstanding job of preparing this submission, which we believe is founded on compelling clinical evidence that supports broad diagnostic utility in the management of prostate cancer.”
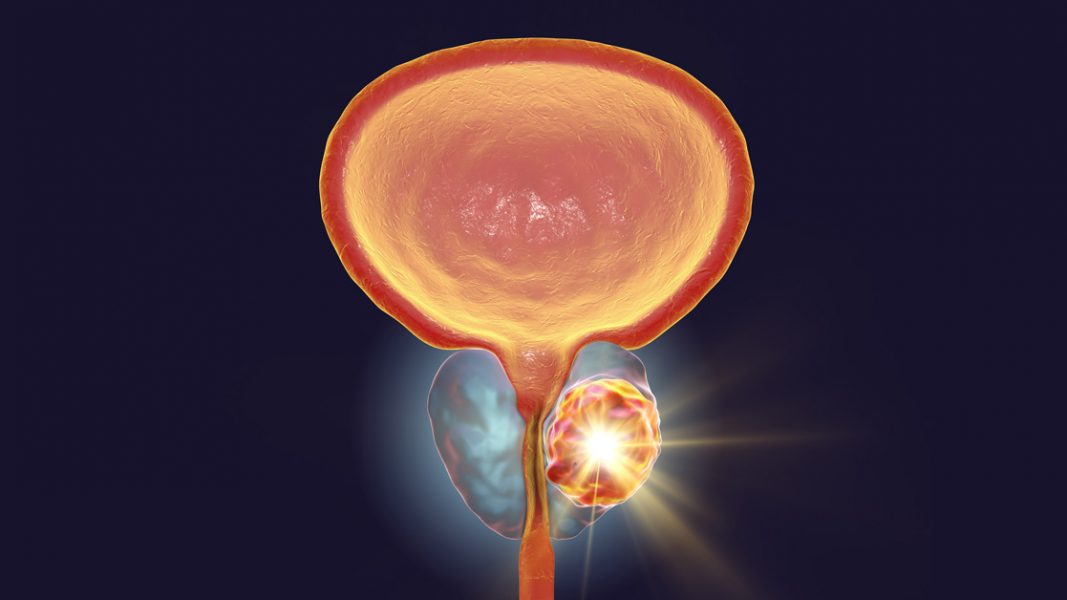
The imaging agent, named TLX591-CDx, is a novel product which targets prostate-specific membrane antigen which can then be identified through radiology. As well as prostate cancer, the molecularly targeted radiation products have potential to identify renal cancer and glioblastoma which light up when a patient undergoes Positron Emission Tomography (PET) testing.
The FDA submission follows on from Telix’s European submission in April 2020 where approval will provide Telix with commercial opportunities in 14 European countries. The Company is expecting the European approvals process to be completed by early 2021.
While awaiting FDA approval, Telix has been advancing its commercialisation pathways, having partnered with Cardinal Health and Pharmalogic in preparation for a product launch.
The global addressable market for prostate cancer imaging is valued at around USD $850 million annually.
- Harris Technology to expand refurbished tech division amid rising demand from cost-conscious Australians - April 30, 2025
- Harris Technology secures major investment from Taiwan’s FSP Technology at 100% premium - March 10, 2025
- ARC Funds acquires 30% of auzbiz Capital as latest direct-to-investor marketing venture - October 8, 2024












Leave a Comment
You must be logged in to post a comment.