Working closer towards commercialising its cutting-edge graphene Biochip, semiconductor company Archer Materials (ASX: AXE) has dispatched its latest chip design iteration to a certified foundry in Spain for fabrication through a whole wafer manufacturing run. This move is part of Archer’s ongoing commitment to pushing the boundaries in both quantum computing and medical diagnostics while expanding its network of foundries that will be able to manufacture their semiconductor chips.
Archer previously validated earlier generations of its Biochip graphene field effect transistor (gFET) designs through successful collaborations with foundries in Germany and the Netherlands. The decision to engage a foundry in Spain for the new design aims to leverage the country’s expertise and facilities, providing Archer with additional insights and benchmarking opportunities.
The chosen foundry in Spain holds ISO 13485 certification, specifically tailored for the manufacturing of medical device components. This certification stands as a significant milestone, underlining the potential for future manufacturing partnerships focused on medical applications—a crucial aspect of Archer’s Biochip development strategy.
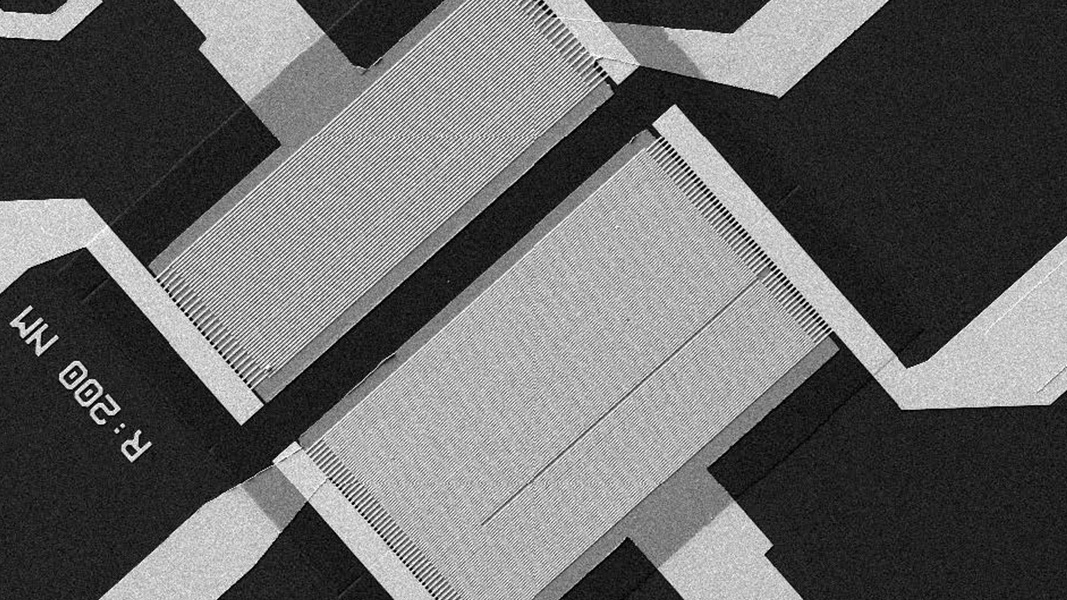
The new Biochip design promises several advancements over its predecessors, particularly in gating and channel definition. These enhancements aim to optimise the structures for liquid multiplexing, expanding the potential applications of the Biochip and enhancing overall quality control.
By collaborating with multiple foundries in Europe, Archer is strategically diversifying its gFET design techniques. This approach increases the Biochip’s potential applications and exposure, as well as reinforces the Company’s ‘fabless’ strategy whereby they do not incur the major capital expense of building their own foundry, instead having direct access to the best tech in the world via external partners.
Each foundry’s capacity and specialist capabilities in semiconductor chip manufacturing, including graphene characteristics, play a crucial role in optimising the gFET design for compatibility and readiness.
The decision to conduct wafer runs in various foundries is essential for fine-tuning the gFET chip development process. It ensures that the Biochip meets the highest standards of quality and compatibility across different manufacturing environments.
Dr. Mohammad Choucair, CEO of Archer, highlighted the importance of the recent advances, stating, “By developing various designs for our Biochip gFET sensor, we are able to widen our foundry network, improve quality control of our chips, and expand possible applications.
“Working with an ISO certified foundry for the manufacture of medical device components aligns with the nature and purpose of our Biochip, which is to potentially transform the medical diagnostics industry by providing better access for the detection of disease.”
The anticipated delivery of the new gFETs in the first half of 2024 marks a crucial milestone for Archer. Upon delivery, Archer will conduct thorough testing of the chips in its Australian laboratory, ensuring that the Biochip meets Archer’s rigorous standards.
The strategic decision to utilise external foundries aligns with a broader financial strategy. By avoiding the costly exercise of building its own foundries, or associated overheads of staffing and regulatory compliance.
Archer is also working with the foundry partner in Spain to integrate testing of the gFET devices at point of manufacture to improve the efficiency of their technology development processes.
This approach allows Archer to tap into the specialised expertise of established foundries worldwide, optimising the Biochip’s development without the burden of extensive infrastructure investments.
With successful validations in Germany and the Netherlands and ongoing collaborations in Spain, Archer is positioning itself at the forefront of innovation, laying the foundation for a transformative future in the Australian semiconductor industry.
For the quarter ended 30 September 2023, Archer reported $2 million in net operating outflows tied to R&D with a cash balance of $21.3 million. Since then, the Company has received a $1.45 million cash rebate from the Australian Federal Government’s Research and Development Tax Incentive program.
- Harris Technology to expand refurbished tech division amid rising demand from cost-conscious Australians - April 30, 2025
- Harris Technology secures major investment from Taiwan’s FSP Technology at 100% premium - March 10, 2025
- ARC Funds acquires 30% of auzbiz Capital as latest direct-to-investor marketing venture - October 8, 2024












Leave a Comment
You must be logged in to post a comment.