If you thought only cars could be autonomous (hey, Tesla), think again. Medical device company Control Bionics (ASX: CBL) has wheeled the technology into autonomous wheelchairs, ensuring people with Amyotrophic Lateral Sclerosis (ALS)—a motor neuron disease—live a better, “freer” life.
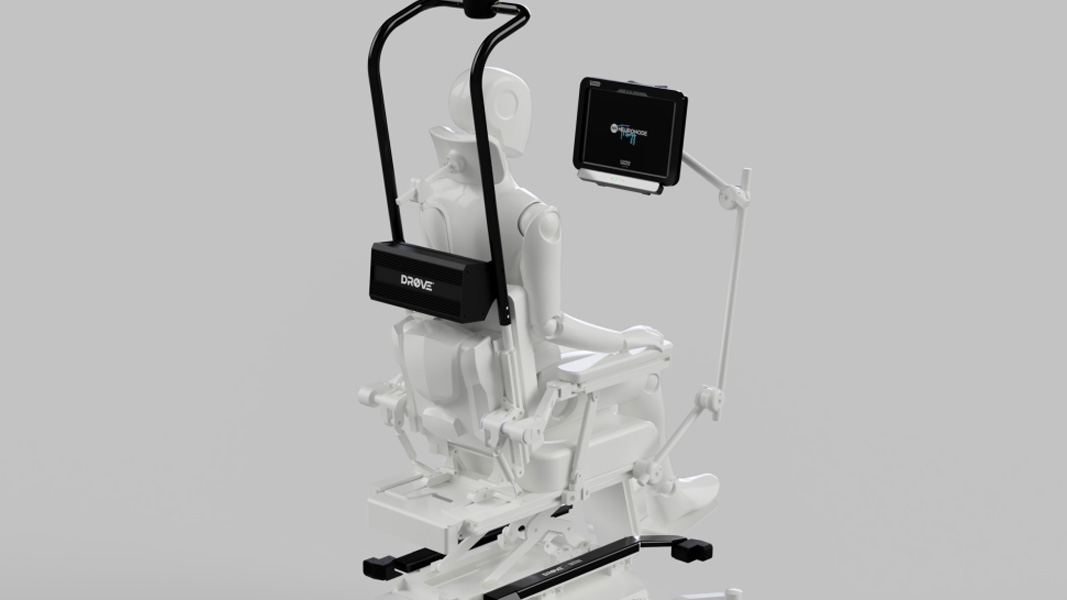
In support of that, the Company’s wholly-owned US subsidiary has secured a research grant of $577.5k from the US-based nonprofit Amyotrophic Lateral Sclerosis Association (ALS Association). This funding, distributed over the next 18 months, aims to advance the development of Control Bionic’s autonomous wheelchair module: DROVE. The technology is set to become the world’s first commercially available autonomous module for power wheelchairs.
The ALS Association’s support is crucial in propelling this advancement into the USA, representing the largest potential market. On average, as per the Centres for Disease Control and Prevention in the US, about 5,000 people are diagnosed with ALS every year.
The ALS Association’s research grant is helping improve the DROVE system for power wheelchairs. This involves checking if it’s easy for technicians and caregivers to use, testing it in real-world situations, exploring new navigation technologies, creating upgrades for different wheelchairs, understanding FDA regulations, and planning how to get funding from other sources. The grant covers costs for Control Bionic as well as external expenses.
CEO Jeremy Steele said, “We are delighted that the ALS Association has awarded us this grant in support of our efforts to bring this groundbreaking technology to the US under the guidance of James Schorey, our CTO and the principal investigator for this grant. Control Bionics remains focused on how we can develop technology to improve the lives of our customers and this award will support and accelerate this objective. The US is a key market for Control Bionics but also represents a large market for our autonomous wheelchair product and we are excited by the potential this award brings us.”
Control Bionics aids those with communication challenges from diseases like Motor Neurone Disease (MND) and Amyotrophic Lateral Sclerosis (ALS). In a similar vein, established in 1985, the ALS Association is the primary nonprofit in the US addressing ALS, providing support, leading global research, and partnering for comprehensive care and potential cures.
On the impact of DROVE, senior vice president of research at the ALS Association, PhD, Kuldip Dave, said, “Limitations with mobility significantly impact the lives of people with ALS. By supporting the development of assistive technologies like this autonomous wheelchair module, we can help people living with the disease move and maintain their independence for as long as possible. This is vital for improving quality of life and for making ALS truly ‘liveable’.”
Individuals with ALS often rely on powered wheelchairs, but conventional models limit independence to joystick use in open spaces. Recognising challenges for those in mid-to-late disease stages, the ALS Association is backing the DROVE autonomous wheelchair module. This self-driving technology enables advanced ALS patients to independently navigate homes or other locations. Users can choose destinations through assistive technologies, and the wheelchair autonomously maneuvers, handling tasks like turning, entering doorways, and avoiding obstacles without constant user input.
Much like a Tesla, DROVE improves driver safety and takes all the workload. For wheelchair users, this could result in a significantly improved quality of life.
- Ovanti’s iSentric signs contracts worth $14.4m with Malaysian commercial bank - June 27, 2024
- Baby Bunting fights back from retail downturn with 5-year strategy, includes Gen-Z focus and self-funded growth - June 27, 2024
- CLEO meets with US FDA to develop strategy for ovarian cancer test launch - June 26, 2024












Leave a Comment
You must be logged in to post a comment.